
Steroid-toxicity
can now be measured
in practice
Practitioners can monitor steroid-toxicity in the background
Practicing physicians have grappled with steroids for decades. In recent years, an explosion of innovative strategies aimed at tapering or even eliminating steroids for immune-mediated inflammatory diseases has offered an array of alternative treatments. Therapeutic pipelines are full of exciting biologics, targeted agents and treatment strategies ready for clinical development.
Essential to adoption of new and approved treatments to help the largest numbers of patients is the ability to equip clinicians to monitor for emerging steroid-toxicity. The core of the Steritas mission is to offer the same scientifically rigorous clinical outcome assessments (COAs) deployed in research, for use in routine clinical settings. Using data routinely collected in the clinic, the GTI-MD can be integrated into electronic medical record (EMR) systems, and equip doctors to detect emerging steroid-toxicity before it is clinically evident in their patients. The GTI-MD works in the background and alerts the physician only when steroid- toxicity rises to a dangerous level. Validated steroid-toxicity scores can support prior authorization of steroid alternatives that are critical to patient well-being.
Essential to adoption of new and approved treatments to help the largest numbers of patients is the ability to equip clinicians to monitor for emerging steroid-toxicity. The core of the Steritas mission is to offer the same scientifically rigorous clinical outcome assessments (COAs) deployed in research, for use in routine clinical settings. Using data routinely collected in the clinic, the GTI-MD can be integrated into electronic medical record (EMR) systems, and equip doctors to detect emerging steroid-toxicity before it is clinically evident in their patients. The GTI-MD works in the background and alerts the physician only when steroid- toxicity rises to a dangerous level. Validated steroid-toxicity scores can support prior authorization of steroid alternatives that are critical to patient well-being.
The STOX Suite: a range of COAs to suit varying needs
Whether the end-goal is to improve clinical outcomes assessment in routine practice or to strengthen the evidence gathered in prospective studies and clinical trials, the STOX® Suite, our collection of clinical outcome assessment (COA), can help you improve the accuracy, reproducibility and efficiency of steroid-toxicity assessment.
- The GTI: calculates change scores across nine health domains in adults
- The GTI-MD: quantifies change across four metabolic domains
- The pGTI: captures the change scores in ten health domains in children
The STOX Suite is the first collection of validated clinical outcome assessments (COAs) of steroid-toxicity. Each COA can be licensed alone or as a bundle for both retrospective and prospective applications. These applications include:
- Clinical trials
- Academic studies
- Health economics and outcomes research (HEOR)
- Real-world experiences
- Clinical practice
The STOX Suite (GTI, pGTI and GTI-MD) has been licensed in 25+ disease indications, across 1100 sites in 80 countries across the world.
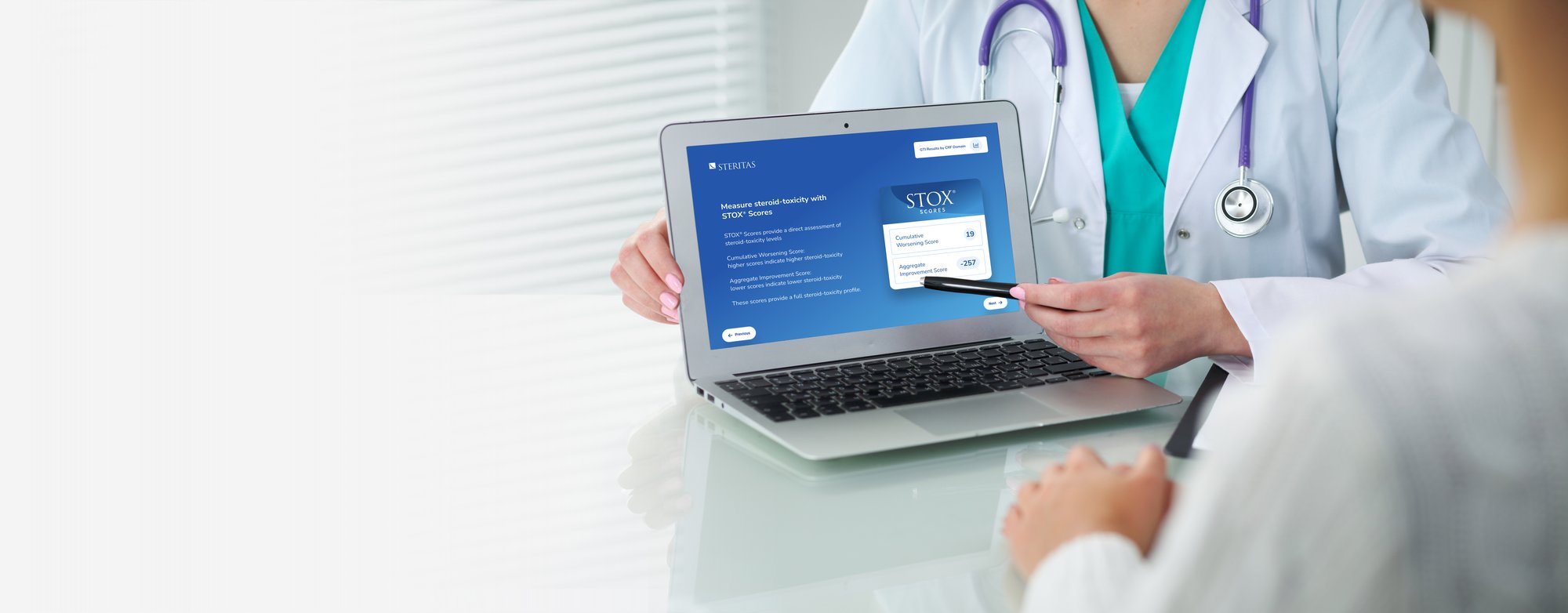
Steritas GTI Integrated into Routine Clinical Practice
Patient-specific differences in steroid-toxicity susceptibility identified
Learn more_600x600pxl.png?width=480&height=480&name=Murray%20Urowitz%2c%20MD%2c%20FRCP(C)_600x600pxl.png)
"If you ask the doctor and patient what they want most of all, their unanimous answer will be not to have to use steroids."
Murray Urowitz, MD FRCP(C)
Professor of Medicine at the University of Toronto
Senior Staff Rheumatologist at the Toronto Western Hospital
Senior Scientist at Krembil Research Institute
Clinical Director, Centre for Prognosis Studies in the Rheumatic Diseases, University Health Network
"Steroid toxicity is rampant and it can be fatal - bringing it onto the radar screen of the practicing physician is really, really important."
James T. Rosenbaum, MD
Chief of Ophthalmology emeritus at the Legacy Devers Eye Institute, Portland, Oregon

"A version of the GTI that could be built into the EPR and generate a big red pop-up that takes up the whole screen and urges providers to record data and to think differently about the prescription would provide the wake-up call that is so often needed."
Michelle Petri, MD MPH
Professor of Medicine at the Johns Hopkins University School of Medicine

"Steroid-toxicity is a huge problem amongst our patients. When they have to be on these drugs for one or more years to keep the disease suppressed they experience many, many complicated side effects, some of which are life threatening."
Dedee Murrell, MD DSc
Head of the Department of Dermatology and St George Hospital, University of New South Wales, Sydney, Australia
Professorial Fellow at The George Institute for Global Health
"Glucocorticoids cause many major side effects such as mental disturbance and gastric ulcers, but we also have to be conscious of the adverse effects - just one milligram of prednisone is harmful to the metabolism."
Yoshiya Tanaka, MD PhD
Professor and Chairman of the First Department of Internal Medicine, School of Medicine. Dean of Graduate School of Medical Science, University of Occupational and Environmental Health, Japan

