
Give sponsors the competitive edge in safety and efficacy
Enhance clinical trials with the first validated clinical outcome assessments of steroid-toxicity
Glucocorticoids have been the cornerstone of treatment of inflammatory diseases for over seven decades. Their ability to rapidly reduce inflammation in a broad range of diseases in the short term must be balanced against the dangers associated with their long term use.
Despite the dozens of known toxicities they cause, glucocorticoids are still the benchmark against which all new therapies are measured. It’s therefore important that clinical trials are able to demonstrate that these therapies can effectively reduce glucocorticoid-toxicity.
The Glucocorticoid Toxicity Index (GTI) is the first validated clinical outcome assessment (COA) that measures glucocorticoid toxicity directly. The GTI and its siblings (pGTI and GTI-MD) provide a powerful advantage for cost-effective product design and evidence-gathering at multiple points in the development cycle, including:
- Target Product Profile (TPP) to specify a product that will be safe, effective and commercially viable
- Patient stratification for clinical trial recruitment efforts
- Critical endpoint in clinical trials to demonstrate drug candidate safety, efficacy and performance relative to alternative treatments
- Risk prediction modeling and treatment monitoring to identify at-risk patients who may benefit from earlier initiation of glucocorticoid-sparing therapies
Incorporating the GTI as an essential trial endpoint can provide a competitive advantage to your drug discovery partners and allow you to differentiate yourself from competitors.
Gain a competitive edge by including the STOX Suite in your eCOA library
The STOX® Suite enables accurate measurement of steroid-toxicity. The STOX Suite includes four clinical outcome assessments (COA) optimized for different settings:
- The GTI: calculates change scores across nine health domains in adults
- The GTI-MD: quantifies change across four metabolic domains in claims and EMR data
- The pGTI: captures the change scores in ten health domains in children
The STOX Suite is the first collection of validated clinical outcome assessments (COAs) of steroid-toxicity. Each COA can be licensed alone or as a bundle for both retrospective and prospective applications. These applications include:
- Clinical trials
- Academic studies
- Health economics and outcomes research (HEOR)
- Real-world experiences
- Clinical practice
Approval of avacopan for ANCA-associated vasculitis underscores the value of the STOX Suite in clinical trials
ADVOCATE was a phase 3 trial comparing oral avacopan with oral prednisone in ANCA-associated vasculitis (AAV).[1] The primary endpoints were clinical remission at week 26 and sustained remission at 52 weeks. ADVOCATE was the first trial to incorporate glucocorticoid-induced toxic effects as a trial endpoint, using the GTI as the most important secondary endpoint for efficacy.
The trial demonstrated noninferiority of avacopan at 26 weeks and superiority at 52 weeks. The GTI measured glucocorticoid-toxicity results using the GTI cumulative worsening score (CWS) and aggregate improvement score (AIS) at 26 weeks, and showed a reduction in toxicity for the avacopan group as follows:
The CWS, and AIS reveal lower steroid toxicity for avacopan vs. prednisone
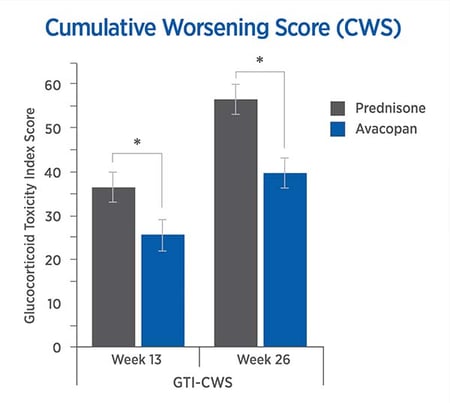
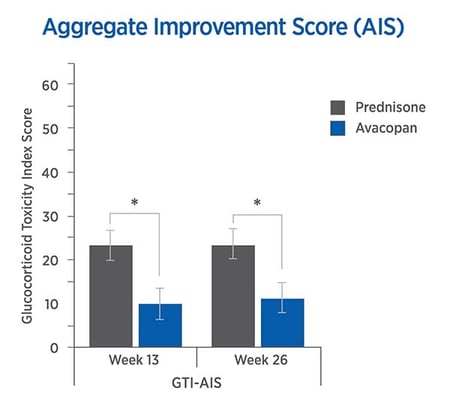
For each trial participant, data were entered into the GTI to generate the Cumulative Worsening Score (CWS), and the Aggregate Improvement Score (AIS), at weeks 13 and 26. The lower scores in the avacopan group confirmed lower steroid-toxicity.
The mean CWS and AIS were both lower in the avacopan group.

"The damage caused by steroid exposure is not surprising - it is expected."
Deborah Gelinas, MD
Neuromuscular Expert Medical Affairs for Argenx

"Trial sponsors have a great opportunity and challenge to demonstrate what they mean by steroid-sparing and how that translates into healthcare benefits such as reduced hospitalizations, and improved quality of life."
Sudhakar Sridharan, MD
Vice President in Medical Science & Strategy Division at PPD/ThermoFisher

"It's very clear that being able to measure things is essential if we want to change them. With the GTI, the pGTI and the GTI-MD, we can measure steroid-toxicity in both clinical trials and clinical practice."
John H. Stone, MD MPH
Professor of Medicine at Harvard Medical School, and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital

"Nobody disputes the value of glucocorticoids, and while most clinicians recognize the damage they cause to patients, they don’t recognize the impact they have on clinical research.”
Andreas Reiff, MD
Senior vice president and global therapeutic area head for inflammation/immunology, Parexel

"The GTI is the first and only quantitative measure of steroid-toxicity - It allows us to quantify the scale of adverse reactions, helps us taper steroids, and look after our patients more effectively."
Wen Zhang, MD PhD
Professor Rheumatology at Peking Union Medical College Hospital (PUMCH), Beijing, China

"The GTI is being used in many trials around the world and the development of the GTI-MD should enable the majority of rheumatologists to quantify glucocorticoid toxicity in day-to-day practice."
Frank Buttgereit, MD
Professor of Rheumatology and Deputy Head of the Department of Rheumatology and Clinical Immunology at the Charite University Medicine (CCM) in Berlin
Surge in adoption of the STOX Suite of Clinical Outcomes Assessments (COA)
In response to substantial investment in research and digitization of its COAs, Steritas has seen a surge in licensing and deployment of the STOX Suite. This reflects the growing recognition of the importance of measuring and monitoring steroid-toxicity in pharmaceutical research and clinical practice respectively.
The Steritas STOX Suite (GTI, GTI-MD, and pGTI) has been used in over 75+ clinical trials, in 1100 sites across 80 countries, underlining the growing impact that quantitative steroid-toxicity assessment is having on clinical development in inflammatory diseases.
Steritas is keen to support studies that align with its goal of reducing the impact of steroid treatment on patients; 90% of applications to date have been successful in accessing GTI instruments for their studies.



