In October 2021, the US Food and Drug Administration issued its first new drug approval for antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) in a decade. The first-in-class approval allows avacopan to be used as an adjunct to standard therapy for the two main forms of ANCA-associated vasculitis - making avacopan the first drug to be approved with ANCA-associated vasculitis as the primary indication.
Any first-in-class drug approval is exciting, but dig a little deeper and what makes this even more exciting is that this is the first instance of a reduction in steroid toxicity, as measured by the Steritas GTI, as the most important secondary efficacy endpoint in the clinical trials.
As John Stone, MD MPH Director of Clinical Rheumatology at Massachusetts General Hospital, professor of Medicine at Harvard Medical School and co-founder of the Vasculitis Center at Johns Hopkins University, explains:
“Steroid toxicity is often seen as part of the wallpaper in the examining room. It’s in the background and gets completely ignored it. We talk about overall side effects in clinical trials, and we talk about the side effects associated with cyclophosphamide, not even bothering to mention the side effects of steroids, which are even worse than cyclophosphamide. More patients with ANCA-associated vasculitis die from steroid-toxicity associated infection than they do from their underlying disease!”
What is ANCA-associated vasculitis?
ANCA-associated vasculitis is a group of progressive, rare, severe autoimmune diseases that affects approximately 150 people per million of the population in Europe 1,2. In the United States, the annual incidence of AAV is 3.3/100,000, with a prevalence of 42/100,000 3. AAV can affect blood vessels in different parts of the body (vasculitis means inflammation of blood vessels), resulting in damage to vital organs such as the lungs, kidneys, nervous system, gastrointestinal system, skin, eyes, and heart 2. The disease causes a lot of suffering to the patients who are affected by it.
This highlights the importance of finding better treatment options. Traditionally, steroids are used for treatment, which have significant side effects. The C5a receptor inhibitor avacopan is being investigated as an alternative treatment option, and it is exciting news that this new steroid-sparing drug has now been approved by the FDA for ANCA-associated vasculitis, which will help improve the quality of life of patients with AAV 4.
The ADVOCATE trial – explanation and results
The ADVOCATE clinical trial was conducted to evaluate whether avacopan could replace a glucocorticoid-tapering regimen used in the treatment of ANCA-associated vasculitis. For this purpose, the study investigated the toxicity of prednisone versus avacopan.
Glucocorticoid-induced toxic effects were a secondary endpoint, as measured by the Stertias GTI 5. For each trial participant, patient data was entered into the GTI to generate the Cumulative Worsening Score (CWS) and the Aggregate Improvement Score (AIS) at study weeks 13 and 26 [click this link for more information about the Steritas GTI and CWS and AIS scores]. A lower score corresponds to lower toxicity for both indices. CWS and AIS revealed statistically and clinically significant differences between treatment groups. The mean CWS and AIS for all trial participants are both statistically significantly lower for avacopan than for prednisone.
Compared to standard prednisone-based treatment, avacopan is superior in terms of sustained remission at 52 weeks. The mean change from baseline in worsening toxicity for eight steroid toxicity ranges was more favorable for avacopan than for prednisone, as shown in Figure 1. Based on these trial results, the FDA has approved avacopan as an add-on therapy for ANCA-associated vasculitis.
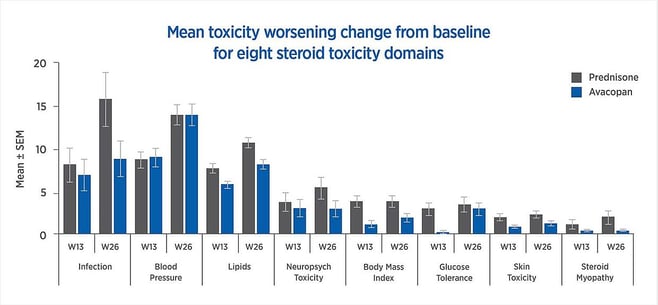
Figure 1: Mean toxicity worsening change from baseline for eight steroid toxicity domains
As described by a paper published in The Journal of Allergy and Clinical Immunology in early 2021, the Minimum Clinically Important Difference MCID is 10 points In the ADVOCATE study, the GTI-CWS difference between avacopan-added and prednisone-only treatment groups at 13 and 26 weeks was -11 and -16.8 points respectively. The GTI-AIS difference between treatment groups at 13 and 26 weeks was -13.3 and -12.1 points respectively. According to the MCID, these values are both statistically significant and clinically important. With this data, the ADVOCATE investigators were able to use the GTI results to conclude that, at 13 and 26 weeks, avacopan reduced glucocorticoid toxicity compared to standard-of-care.
Conclusion
Treatment with avacopan results in a reduction in toxicity in all but one of the steroid-toxicity domains used in the GTI. Considering how severe steroid-related side effects are in many patients, it is clear how important the secondary endpoint of reducing steroid toxicity is in improving patient outcomes. Therefore, the investigators of the avacopan trial reported GTI as the most important secondary efficacy endpoint 4,6.
Download and read this important publication for the treatment of ANCA-associated vasculitis here.
References
1 Myancavasculitis https://www.myancavasculitis.com/
2 Understand AAV https://understandaav.com/
3 Berti A, Cornec D, Crowson CS, Specks U, Matteson EL. The Epidemiology of ANCA Associated Vasculitis in the U.S.: A 20 Year Population Based Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/the-epidemiology-of-anca-associated-vasculitis-in-the-u-s-a-20-year-population-based-study/. Accessed May 10, 2022.
4 Jayne DRW, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group. Avacopan for the Treatment of ANCA-Associated Vasculitis. N Engl J Med. 2021 Feb 18;384(7):599-609. http://doi.org/10.1056/NEJMoa2023386.
5 John H. Stone, P. Jane McDowell, David R.W. Jayne, Peter A. Merkel, Joanna Robson, Naomi J. Patel, Yuqing Zhang, Huibin Yue, Pirow Bekker, Liam G. Heaney, The glucocorticoid toxicity index: Measuring change in glucocorticoid toxicity over time, Seminars in Arthritis and Rheumatism, Volume 55, 2022, 152010, ISSN 0049-0172,
https://doi.org/10.1016/j.semarthrit.2022.152010.
