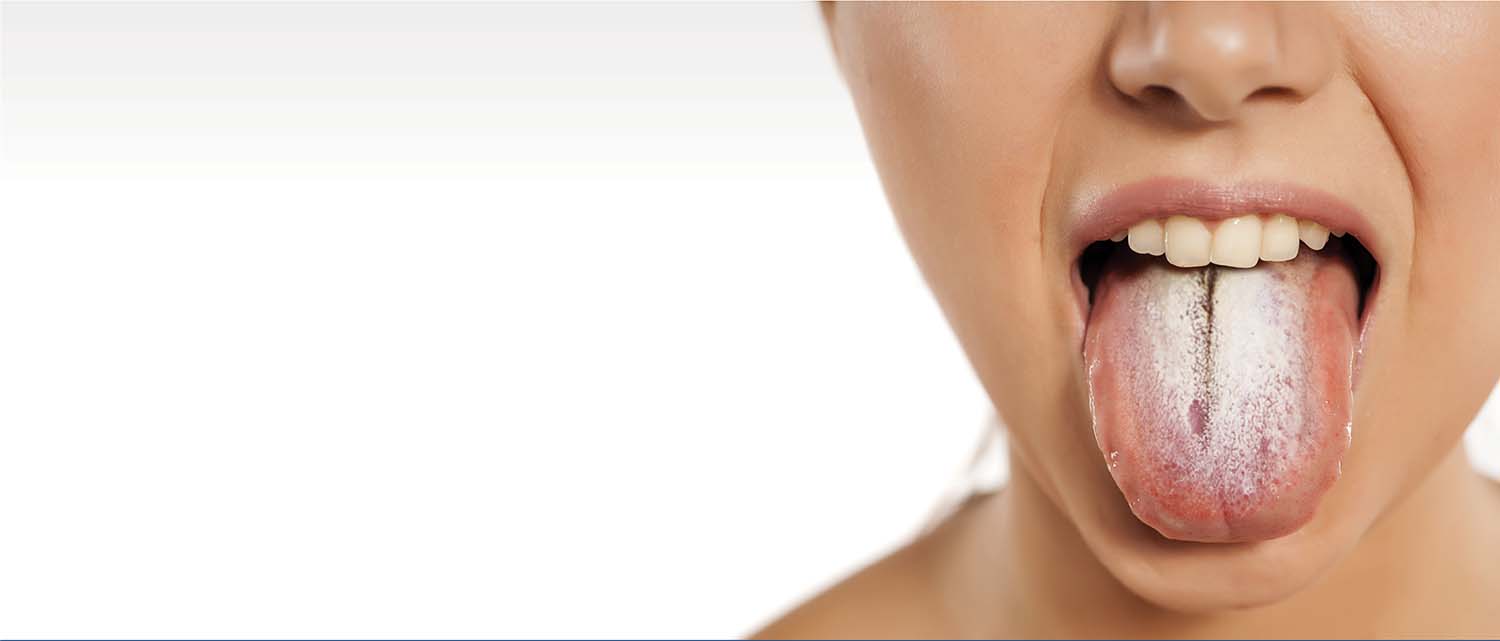
We are optimizing the use of steroids
Track to taper now
At Steritas, we understand the devastating impact that steroid treatment can have on patients around the world.
Tracking steroid-toxicity with the STOX® Suite of clinical outcome assessments provides clinicians with the means to taper steroid usage, optimize treatment and improve outcomes for patients.
Our expanding suite of clinical outcome assessments is driving the development of new steroid-sparing therapies through their use in clinical trials. To date, the suite has been used in over 80 clinical trials around the world.


Our Vision
A worldwide steroid taper
Our Mission
To shift steroid prescribing patterns for the benefit of patients, by measuring steroid-toxicity in research and in practice.
We are clinical investigators improving clinical investigation
One of the goals of developing new steroid-sparing therapies for inflammatory disease is to minimize glucocorticoid-induced side-effects. Critical to the success of clinical studies is the ability to accurately measure both the magnitude of steroid-toxicity and the degree of change in patients on different therapeutic protocols.
The Steritas GTI was developed by an international group of 20 clinical investigators to address this unmet need. Their combined expertise in inflammatory diseases encompassed rheumatology, pulmonary medicine, nephrology, neurology, psychiatry, dermatology, ophthalmology, pharmacology, maternal-fetal medicine, and infectious disease. The GTI was developed using data-driven analysis, expert consensus, and multi-criteria decision informatics.
Martha N. Stone
Chief Executive Officer, Steritas
Ms. Stone, the CEO of Steritas, has decades of experience as a professional strategist advising leaders across a range of industries in the US.
Ms. Stone, the CEO of Steritas, is driven by the scope of the public health problem addressed by Steritas and the opportunities to scale the company’s technologies to improve the quality of care for millions of adult and pediatric patients worldwide. She envisions the deployment of Steritas technologies in clinical trials, at point of care, and with a global public health initiative.
In founding Steritas, she recognizes that the company’s core technology - the Steritas GTI - has met the moment. Conservative estimates place the number of individuals taking long-term glucocorticoids at more than 50 million adults (1% of the world’s population). She has assembled the Steritas team to license and leverage the technology to improve health across all steroid-dependent diseases. This undertaking draws on both individual patient experience and global physician expertise to improve the lives of men, women, and children whose treatment still depends heavily on steroids. To contribute to the creation of new therapeutic strategies and treatment pathways that reduce steroid toxicity is a gratifying way to serve the public.
Martha, driven by the thrill of enterprise and a love of language, is a business and brand strategist at heart. She has helped design and execute global manufacturing strategies with companies such as Hewlett-Packard and Autodesk and consulted with the leaders in a range of mid-sized companies seeking to differentiate their service offerings to grow market share.
As Communications Director of American Promise in its start-up phase, she worked to drive the adoption of the 28th Amendment to the United States Constitution, based in rare national, bi-partisan consensus that people – not big money, not large corporations, not powerful unions, and not special interests – must govern themselves.
Stone earned a degree in English from Williams College where, with a wink at the school mascot – the Purple Cow – she built and branded her first successful business: Holey Cow Mending, a thymbol of quality. A later enterprise, launched while the mother of young children, was Perpetual Cow Productions. That company created a best-selling audio CD called for crying out loud TM unlikely sounds to calm your baby. The collection of white noise to soothe fussy infants that sold online, in independent shops, and through large chain stores, was also included in a state-wide program to equip 5000 new parents with coping strategies to help prevent shaken baby syndrome. for crying out loud garnered attention from The Wall Street Journal, NPR’s Morning Edition, and a variety of parenting media channels.
From the whimsical to the great challenges in public health, Martha Stone understands the way people and business operate, and her body of work has been to create strategies that attract, build and secure community in support of good works.

John H. Stone, MD MPH
Chair, Scientific Advisory Board
Dr Stone, a rheumatologist, is a Professor of Medicine at Harvard Medical School and the Edward A. Fox Chair in Medicine at the Massachusetts General Hospital.
Dr Stone graduated from Harvard Medical School, completed his internal medicine training at Johns Hopkins University School of Medicine, and trained in rheumatology at the University of California-San Francisco. Before being recruited to Mass General, he co-founded and directed the Vasculitis Center at Johns Hopkins. His work focuses on vasculitis, a group of inflammatory diseases that target blood vessels, as well as on a newly-described multi-organ condition known as IgG4-related disease (IgG4-RD).
Dr Stone led randomized, double-blind, placebo-controlled trials in both ANCA-associated vasculitis and giant cell arteritis. The results of those trials led directly to the worldwide approval of new treatments for those diseases.
In the early days of the COVID pandemic, Dr Stone developed and led a decisive trial of an anti-cytokine therapy, the BACC Bay Trial, across 7 Boston hospitals. He has assembled a creative research group to tackle IgG4-RD, a condition largely unknown in the U.S. before his arrival at Mass General in 2008. He has hosted or co-hosted four international symposia on IgG4-RD, designed and conducted clinical trials of novel medications for this condition, and fostered collaborations with the Ragon Institute in Cambridge, Massachusetts that have led to new insights into human immunology.
Since 2015, drawing on 25 years of experience with glucocorticoid treatments for his own patients, Dr Stone has focused on the measurement and prevention of glucocorticoid toxicity. He organized the international group of experts to create the Glucocorticoid Toxicity Index (GTI) in adults and a second collaboration among pediatricians to develop the pediatric version of the instrument, the pGTI.
Dr Stone seeks to facilitate the transition of treatment for patients with inflammatory diseases such into an era of safe, highly-effective, steroid-sparing medications.

Lili Brilstein
Advisory Board
Lili Brillstein is a leader in value-based specialty care, founder and CEO of BCollaborative, and has significantly influenced specialty care models in the US.
Lili Brillstein is an internationally recognized thought leader in the advancement of Episodes of Care as a value-based approach to specialty care.
As the founder and CEO of BCollaborative, she fosters partnerships among payers, providers, start-ups and community organizations to support care models that emphasize collaborative, outcome-focused and resource-efficient healthcare delivery. Her work at BCollaborative aims to enhance patient outcomes and experiences while ensuring the optimal use of resources through innovative partnerships.
Previously, Brillstein played a pivotal role at Horizon BCBS of New Jersey, leading the Episodes of Care program to become the largest and most advanced value-based care initiative for specialty care in the United States. Her contributions extend to academia and policy development; she has been a guest lecturer at Harvard Business School, worked with esteemed professionals such as Michael Porter, and co-authored publications on value-based care. She has collaborated with CMMI and PTAC on specialty care models and has served on the Advisory Boards of the US Women’s Health Alliance and the Quality Cancer Care Alliance; both national coalitions focused on advancing value-based care to improve quality and cost of care delivery. She is a former Adjunct Associate Professor at The Rutgers School of Pharmacy and a member of the Board of Directors for the NJ Coalition to End Domestic Violence.

Mike Broxson, MSPH MBA
Advisory Board
Mike Broxson is CEO of Tennyson Advisory LLC, a consulting firm providing biopharma client business and corporate development strategy, and transactions advisory services.
Mike Broxson is CEO of Tennyson Advisory LLC, a consulting firm providing biopharma client business and corporate development strategy, and transactions advisory services. Mike has 25 years of biopharma leadership experience across operations, finance, strategy, new product planning, alliance management, and corporate development.
Previously he was President and CEO of Q32 Bio, a Cambridge, MA, based biotechnology firm focused on targeted therapeutics for diseases of the innate and adaptive immune systems. Prior to Q32 Bio, Mike was Chief Business and Operating Officer at Goldfinch Bio. In that role he built the G&A functions, oversaw fundraising efforts, and closed strategic transactions with Gilead and Takeda. Mike spent 16 years at Takeda Pharmaceuticals, most recently as vice president and global head of R&D business development. There he led or played a significant role in over 75 transactions exceeding $25 billion in deal value, including the acquisitions of Millennium, Nycomed, and Envoy Therapeutics. He initiated Takeda’s externalization practice, establishing new ventures and innovative alliances to support R&D assets.
Mike began his career as a toxicologist for CH2M Jacobs, a global engineering consulting firm. He holds a BA in Economics, and an MSPH, in Toxicology from Tulane University, as well as an MBA from the University of Chicago, and is a CFA® charterholder. He currently serves as an advisor to Life Science Cares and is a former board member of Molecular Templates Inc.

Paul Brunetta, MD
Advisory Board
Dr Paul Brunetta is a physician and clinical researcher and is currently the Head of Clinical and Translational Science at Sana Biotechnology which is advancing cell and gene therapies into the clinic.
Dr Paul Brunetta is a physician and clinical researcher with 14 years of clinical care experience and 22 years of experience in the biotechnology industry and drug development He is a graduate of Johns Hopkins University in Baltimore and Tufts University School of Medicine in Boston. He went to UCSF at the height of the AIDS epidemic and did his internship and medical residency at UCSF. Paul was Chief Medical Resident at San Francisco General Hospital (1993-1994) and then did his Pulmonary and Critical Care Fellowship at UCSF before joining faculty focusing on lung cancer, tobacco-related lung disease, and smoking cessation. He co-founded the Fontana Tobacco Treatment Center and has dedicated significant volunteer time to support FTTC expansion within the UCSF clinical system over the years. He recently co-authored the book Learning To Quit- a comprehensive guide to smoking cessation. Paul is a co-founder of Mobile Applications for Connected Health, a private company developing a comprehensive smoking cessation platform undergoing clinical research at the University of Colorado mHealth Impact Lab in the School of Public Health.
Paul joined the biotech industry in 2002 and has supported the approval of multiple monoclonal antibody therapies and has significant experience interacting with the FDA and EMA in the drug approval process. He has designed, monitored, and implemented trials in autoimmune drug development spanning phase I-IV trials across multiple diseases in neurology, nephrology, rheumatology, immunology, pulmonary medicine, and solid organ transplantation. His clinical research work has been published in the New England Journal of Medicine and other international specialty journals. Paul directed the Genentech Clinical Research Fellowship for 12 years, mentoring fellows across multiple disease areas. Paul joined Juno Therapeutics as SVP, Head of Translational Science in CAR-T development. He’s now Head of Clinical and Translational Science at Sana Biotechnology which is advancing next-generation cell and gene therapies into the clinic.

Dan Hawcutt, MD MRCPCH
Advisory Board
Dan Hawcutt, MD MRCPCH is a Reader in Pediatric Pharmacology at the University of Liverpool, an honorary consultant in pediatrics at Alder Hey Children’s Hospital, and the Director of Alder Hey’s early-phase clinical research facility.
Dr Hawcutt is a Reader in Pediatric Pharmacology at the University of Liverpool and an honorary consultant in pediatrics at Alder Hey Children’s Hospital. He is Director of Research at Alder Hey Children's Hospital, and Director of the NIHR Alder Hey Clinical Research Facility (CRF).
Dr Hawcutt is also chair of the Royal College of Paediatrics and Child Health (RCPCH) / Neonatal and Paediatric Pharmacists Group (NPPG) joint standing committee on medicines, and a member of the Medicines and Healthcare Regulatory Agency Pharmacovigilance Expert Advisory Committees on both Pharmacovigilance and Paediatric Medicines.
In addition, he is chair of the RCPCH training committee for Paediatric Clinical Pharmacology. His research interests include Adverse Drug Reactions, Pharmacogenomics, Pharmacovigilance methods and Early Phase Clinical Trials.

Noreen R. Henig, MD
Advisory Board
Dr Henig is a seasoned physician and biotech leader with extensive experience in lung disease, transplant programs and drug development, holding executive roles in various medical and biotech organizations.
Dr Henig has built a distinguished career as a physician, scientist and executive in the healthcare industry. She is a graduate of Yale University and received her MD with a distinction in Immunology from the Albert Einstein School of Medicine. She completed her internship and medical residency at the University of California, San Francisco during the peak of the AIDS crisis, an experience that underscored the critical need for quality care and effective therapeutics. Her fellowship at the University of Washington further honed her expertise in Pulmonary, Critical Care and Allergy/Immunology, particularly focusing on T-cell function and leukotrienes.
Her notable contributions include leading the Lung Transplant Program and the Adult Cystic Fibrosis Center at Stanford University. She also worked at California Pacific Medical Center, where she supported the transplant ICU and directed the Pulmonary Fellowship program. Transitioning to the biotech industry, Henig played a vital role at Gilead Sciences in pulmonary and cardiovascular development, encompassing clinical trial phases, medical affairs and business development.
As Chief Development Officer at ProQR, she was instrumental in transitioning the company from private to public status. She then became the Chief Medical Officer at Breath Therapeutics, leading to the company's acquisition. At Kezar Life Sciences, she focused on developing treatments for autoimmune diseases and oncology. Currently, Dr Henig serves on the Board of Avidity Biosciences and is pleased to support Steritas's mission to enhance patient health through improved medication use.

David Jayne, MD
Advisory Board
Dr David Jayne is a clinical nephrologist practicing in Cambridge, UK where he directs a severe inflammatory disease service that has supported more than 5,000 patients (mainly vasculitis and lupus) over the last 20 years.
Dr David Jayne is a clinician practicing at Addenbrooke’s Hospital, Cambridge, UK where he directs a severe inflammatory disease service that has reviewed over 5000 patients, mainly vasculitis and lupus, over the last 20 years. He currently holds the position of Professor of Clinical Autoimmunity, Department of Medicine, University of Cambridge. His research group has conducted many first into disease clinical trials in vasculitis and lupus and associated clinical epidemiology and biomarker studies.
Dr Jayne has contributed to 480 peer-reviewed publications and authored 54 book chapters. He has a Google Scholar H Index of 121.
He has led the development of international collaborative networks in vasculitis that have supported larger scale clinical trials, genomic studies, recommendation statements, and disease classification. He is the founder and President of the European Vasculitis Society and a founder member of the Lupus Nephritis Trials Network. He has led or contributed, with academia and industry, to successful Phase III programs for rituximab and avacopan (anti-C5aR) in ANCA vasculitis, mepolizumab (anti-IL5) in eosinophilic granulomatosis with polyangiitis (EGPA), mycophenolate mofetil and voclosporin in lupus nephritis.
Dr Jayne received his BA and MB BChir from Cambridge University and has held research fellowships at the Royal Postgraduate Medical School, London, the School of Clinical Medicine, Cambridge, and the Gonville and Caius College, Cambridge. He was a Clinical Fellow at Harvard Medical School, Boston, MA, and a Clinical Lecturer at the Department of Medicine, Cambridge, UK. He was appointed as Senior Lecturer at St George’s Hospital Medical School, Consultant at Addenbrooke’s Hospital, Cambridge before being appointed a Reader in the Department of Medicine, University of Cambridge, and in 2017 was named Professor of Clinical Autoimmunity, Department of Medicine, University of Cambridge.

Zuwen Kuang
Advisory Board
Zuwen Kuang is a skilled and experienced digital healthcare executive with a proven track record of success in the field of data and analytics.
Zuwen Kuang is a skilled and experienced digital healthcare executive with a proven track record of success in the field of data and analytics.
As the Global Head of Data and Analytics at Fresenius Medical Care, she leads a team responsible for building enterprise big data platforms, delivering advanced analytics solutions and digital interoperability capabilities. This enables the transformation of healthcare data into intelligence that predicts trends and reveals actionable insights that influence long-term business growth.
Zuwen is passionate about the development and implementation of data-driven solutions that enable precision medicine and value-based care.
She holds a Bachelor’s degree in computer science from Anhui University, China, and a Master’s degree in computer science with a focus on game theory from Eastern Kentucky University, US.

Michelle Petri, MD MPH
Advisory Board
Michelle Petri, MD MPH is Professor of Medicine and Rheumatology at Johns Hopkins. She is the Director of the Hopkins Lupus Cohort, a longitudinal study of morbidity and mortality in SLE, and Co-Director of the Hopkins Lupus Pregnancy Center.
Michelle Petri, MD MPH is a Professor of Medicine in the Division of Rheumatology and Director of the Lupus Center at the Johns Hopkins University School of Medicine and Johns Hopkins Hospital in Baltimore, MD.
She earned her medical degree from Harvard Medical School in Boston, MA, and completed her internship and residency in Internal Medicine at Massachusetts General Hospital. Thereafter, Professor Petri completed her Postdoctoral Fellowship in Allergy, Immunology and Rheumatology at the University of California, San Francisco. She subsequently earned her master’s in Public Health and Epidemiology from Johns Hopkins University Bloomberg School of Public Health.
Professor Petri’s research focuses on several aspects of systemic lupus erythematosus (SLE), including atherosclerosis, antiphospholipid syndrome (APS), lupus nephritis and pregnancy. She is the founder and Director of the Hopkins Lupus Cohort, a longitudinal study of the incidence and pathogenesis of thrombotic events and coronary artery disease in SLE. She is also the Co-Director of the Hopkins Lupus Pregnancy Center, a Master of the American College of Rheumatology, and was Chair of the Lupus Now Education Program.

Andreas Reiff, MD
Advisory Board
Andreas Reiff, MD, is the SVP and global therapeutic area head for Inflammation/Immunology at Parexel, with extensive experience in drug development, rheumatology and immunology, and holds several academic and advisory roles.
Andreas Reiff, MD is the Senior Vice President and global therapeutic area head for Inflammation/Immunology at Parexel International Global Medical Services, a global Contract Research Organization (CRO) based in Boston, MA.
He currently oversees drug development in the areas of rheumatology, allergy, dermatology, gastroenterology and ophthalmology and leads a team of 32 subject matter experts.
Before joining Parexel, Dr Reiff was the Chief Medical Officer at TheraKine Inc., specializing in innovative biodegradable polymer technology for drug delivery within the eye. He is also an Adjunct Professor at the Oregon Health Science University, Emeritus Professor at the Keck School of Medicine at the University of Southern California, and the former Division Head of the Division of Rheumatology at Children's Hospital Los Angeles. He was also the director of the Pediatric Rheumatology Program at Miller Children’s Hospital in Long Beach, CA, and a Visiting Clinical Associate Professor at the University of Nevada School of Medicine’s Department of Pediatrics.
Dr Reiff's career began with a medical degree from the University Medical School in Freiburg, Germany, followed by various residencies and fellowships focused on internal medicine, surgery and pediatric rheumatology. He has led numerous clinical trials, acted as a consultant for pharmaceutical companies, and shared his expertise through publications and lectures worldwide on rheumatology and biologic drug development.
A member of various rheumatology societies and advisory boards, Dr Reiff is recognized internationally for his contributions to chronic inflammatory eye disease treatment. He is board-certified in pediatrics and pediatric rheumatology and has been honored with several awards for his work in the field.

Lisa Christopher-Stine, MD MPH
Scientific Advisory Board
Dr Christopher-Stine is a Professor of Medicine and Neurology and the Director of the Johns Hopkins Myositis Center. She currently serves as the Deputy Director of Telemedicine in the Division of Rheumatology and seeks to utilize this outreach effort to extend both clinical and research care in the future.
Dr Christopher-Stine graduated Cum Laude with a B.A. in chemistry from Franklin and Marshall College where she was elected to the Phi Beta Kappa Honor Society. She was elected to the Alpha Omega Alpha medical honor society at Hahnemann University School of Medicine, where she received her MD degree. She attained her Master of Public Health degree from the Johns Hopkins Bloomberg School of Public Health. Her internship and residency training in Internal Medicine were completed at MCP Hahnemann University, where she also served as Chief Resident. She pursued rheumatology fellowship training and joined the faculty of the Division of Rheumatology at Johns Hopkins University in 2003 and became a full professor in 2022.
She serves as a board member of the Institutional Review Board at the Johns Hopkins University and she is one of 24 core faculty members to teach in the Johns Hopkins Medical School Colleges Advisory Program, which provides clinical skills instruction in the first year of medical school and continued career advising throughout all four years of medical school.
As a clinician-scientist, she co-developed and is the Principal Investigator for the Myositis Database, currently numbering well over 2000 patients recruited worldwide. She has a strong interest in patient-reported outcomes and has been the co-chair of an international effort through the OMERACT organization to develop a novel patient-reported outcome measure.

Neelam Goyal, MD
Scientific Advisory Board
Dr Goyal specializes in the diagnosis, management, and electrophysiological testing of neuromuscular disorders (including SFEMG), with expertise in immune-mediated disorders (myositis, myasthenia gravis, CIDP, and vasculitis) and ALS.
Dr Goyal is a Clinical Associate Professor in Neurology and Neurological Sciences at Stanford and specializes in the diagnosis, management, and electrophysiological testing of neuromuscular disorder (including SFEMG), with expertise in immune-mediated disorders (myositis, myasthenia gravis, CIDP, and vasculitis) and ALS. Her research interests include the monitoring and management of short and long-term toxicity of immunosuppressive agents. She also provides botulinum toxin for the treatment of sialorrhea in ALS patients.
Dr Goyal completed her medical school education at SUNY Downstate in Brooklyn, NY. She then finished her neurology residency including her chief year followed by a fellowship year in neurophysiology with a focus on neuromuscular disorders and EMG nerve conduction studies at Stanford University Medical Hospital.
After graduation, she joined the faculty of Stanford University School of Medicine in 2012 as a Clinical Assistant Professor of Neurology and Neurological Sciences in the division of Neuromuscular Medicine. She took on the position of co-Director of the Muscular Dystrophy Association/ALS Clinic in 2016. In 2020, she was promoted to Clinical Associate Professor. She serves on multiple committees within Stanford, including the Clinical Assistant Professor Appointment and Promotions Committee and Health Information Management Committee, as well as multiple committees within the American Association of Neuromuscular and Neurodiagnostic Medicine (AANEM).

Yoshiya Tanaka, MD PhD
Scientific Advisory Board
Professor Yoshiya Tanaka, MD, PhD is Professor and Chairman of the First Department of Internal Medicine, School of Medicine and Dean of Graduate School of Medical Science, University of Occupational and Environmental Health, Japan.
Professor Tanaka has authored and reviewed nearly 600 publications. His scientific focus lies on pathological analysis and development of novel treatments for systemic autoimmune diseases, rheumatic diseases, and osteoporosis. He is the president of the Japanese Association of Clinical Immunology and past president of Japanese Society of Bone and Mineral Research (JSBMR).
He is a director and board member of the Asia Pacific League of Associations for Rheumatology (APLAR), Japanese Society of Internal Medicine, Japanese College of Rheumatology (JCR), Japanese Society of Inflammation and Regeneration.
He is also editor-in-chief of Modern Rheumatology Case Reports, an associate editor of Rheumatology, Cytokine, Arthritis Care and Research, Arthritis Research & Therapy, Inflammation Research, International Journal of Rheumatic Diseases, an editorial board member of Annals of Rheumatic Diseases, RMD Open, Modern Rheumatology, and Inflammation Research. He has received numerous awards from the Japanese Society of Inflammation, JSBMR, JCR, and EULAR.

Wen Zhang, MD PhD
Scientific Advisory Board
Dr Wen Zhang is a Professor in the Rheumatology Department at Peking Union Medical College Hospital (PUMCH), Beijing, China and has been working as a physician for 29 years.
Dr Zhang has focused on IgG4-related disease since 2011. Her expertise encompasses IgG4-RD, Sjogren’s syndrome, Systemic lupus erythematosus, systemic vasculitis, antiphospholipid syndrome, SAPHO syndrome. She also participated in international consensus guidelines on the management of IgG4 related-disease, and the 2019 ACR/EULAR classification criteria for IgG4-RD.
Dr Zhang received her Ph.D. degree in Rheumatology, Department of Rheumatology, Peking Union Medical College, Beijing, China, and her M.D. degree in Medicine, West China Medical University, China

Events
ACR Convergence. November 10-15, 2023
John H. Stone, MD MPH and team will present exciting early results from a study assessing longitudinal steroid-toxicity as measured by the GTI.
IgG4ward Launch Event at ACR Convergence
IgG4ward is dedicated to IgG4-RD advocacy, research and support. Its founder, John H. Stone, MD MPH will attend the launch event.